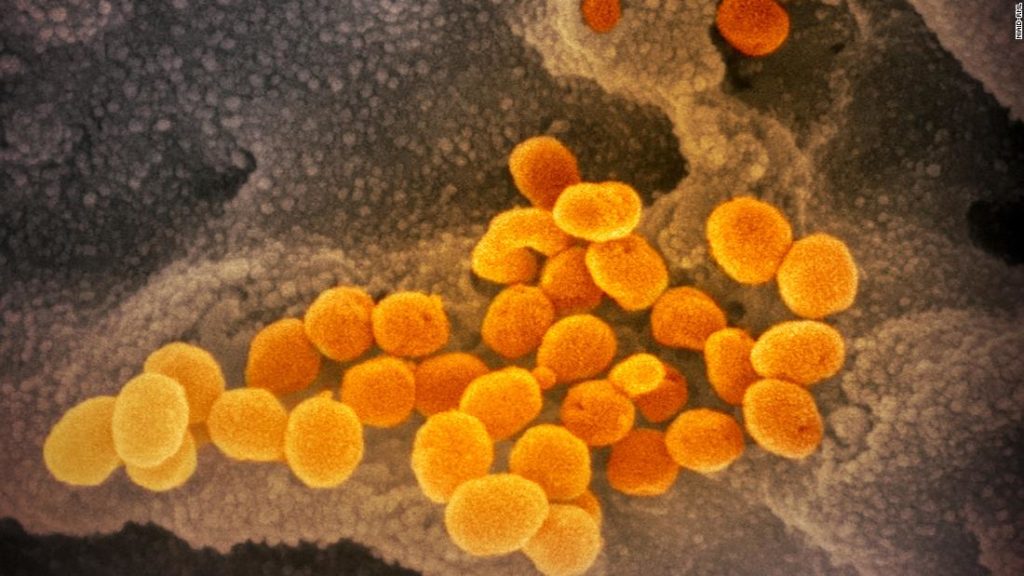
The UK government has announced a “significant first step” in getting the AstraZeneca vaccine “approved for deployment.”
On Friday, it formally referred AstraZeneca’s experimental vaccine candidate to the UK’s medicines regulator for assessment. The British-Swedish drugmaker is developing the vaccine in conjunction with the UK’s Oxford University.
If it gains regulatory approval, the UK will be one of the first countries in the world to receive it, according to the country’s Department of Health and Social Care (DHSC).
AstraZeneca expects to have up to 4 million doses ready for the UK by the end of the year, with 40 million more by the end of March, it added.
The news has arrived at a critical moment for the company, which has been pushing back against criticism about a lack of transparency behind its data.
On Monday, AstraZeneca announced that its vaccine had shown an average efficacy of 70% in large-scale trials.
In one group, 2,741 participants received a half-dose of the vaccine and then a full dose at least a month later. This group was 90% protected against Covid-19.
In the second group, 8,895 participants received a full dose followed by another full dose at least a month later. This group was only 62% protected.
That’s why AstraZeneca says their vaccine is 70% effective, on average.
But some scientists are questioning why the company would report on a pooled result of two different trials, as it deviates from standard reporting on clinical trials.
And in the days following AstraZeneca’s announcement, another point of confusion emerged: a lab error was the reason why some volunteers had received a smaller dose.
In a call with reporters on Wednesday, the US vaccine czar Moncef Slaoui said that the group that got the mistakenly lower dose that yielded the 90% efficacy had been a younger group, with no one older than 55.
That could potentially affect the strength of AstraZeneca’s findings, given that young people typically produce stronger immune responses to vaccines.
In a statement on Friday, the UK Medicines and Healthcare products Regulatory Agency Chief Executive Dr. June Raine said that the body will “rigorously assess the latest data and evidence to be submitted of the vaccine’s safety, quality and effectiveness.”
“The safety of the public will always come first. Our role is to work to the highest standards and safety is our watch word,” she added.
You may also like
-
UK coronavirus variant has been reported in 86 countries, WHO says
-
NASA technology can help save whale sharks says Australian marine biologist and ECOCEAN founder, Brad Norman
-
California Twentynine Palms: Explosives are missing from the nation’s largest Marine Corps base and an investigation is underway
-
Trump unhappy with his impeachment attorney’s performance, sources say
-
Lunar New Year 2021: Ushering in the Year of the Ox