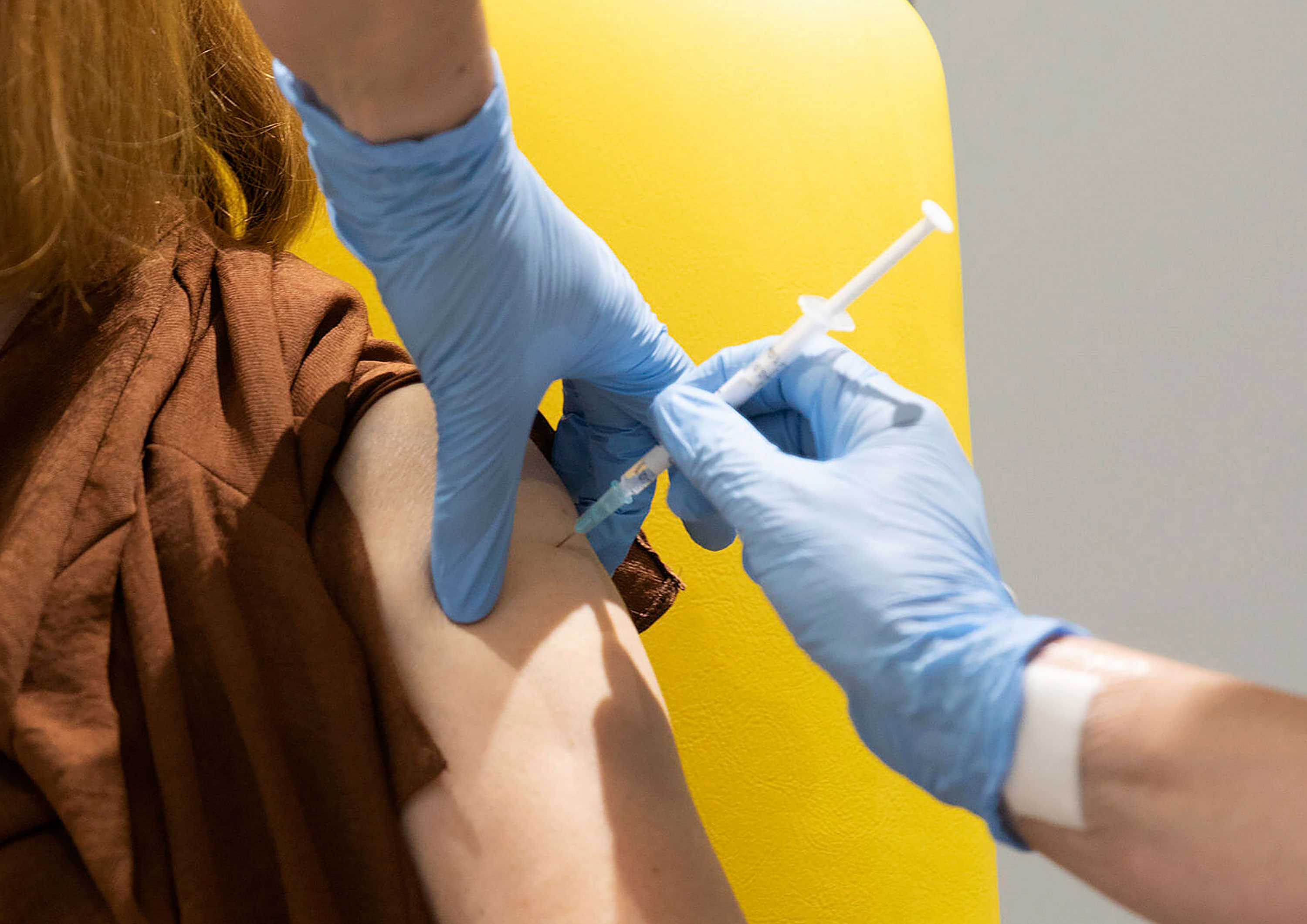
A handful of countries have announced their coronavirus vaccine distribution plans, with some prepping for as early as mid-December.
Here’s where each stand:
AUSTRIA:
Austria became the latest country to announce a plan for distribution when Chancellor Sebastian Kurz announced today that the country was hoping to roll out the vaccine to the elderly, carers and medical workers by January.
Speaking at a press conference in Vienna, Kurz thanked the European Commission President Ursula von der Leyen for procuring vaccines for European countries, calling them a “game changer.”
The European Union has signed deals for the supply of millions of vaccine doses with multiple drugmakers, including AstraZeneca, Moderna and Pfizer.
Kurz said he believes that Austria will return to “normalcy” by next summer.
ITALY:
On Monday, Italy also announced that it hoped to start distributing the vaccine by the end of January.
Speaking on the on Italian television channel La7 on Monday, Italian Prime Minister Giuseppe Conte said the vaccine will be available first to the “fragile and most exposed to danger.”
Conte also said that the vaccine would be administered on a voluntary basis for now.
When asked if he would get vaccinated, Conte said he “will definitely do it,” because when it will be distributed it will be “absolutely safe”.
GERMANY:
German Health Minister Jens Spahn is optimistic a vaccine could be available by December.
“There is reason to be optimistic that a vaccine will be approved in Europe this year. And then we can start with the vaccinations immediately,” Spahn said on Monday, according to CNN affiliate NTV.
Spahn also said that he had asked the country’s 16 regional states to establish immunization centers by mid-December in anticipation of the vaccine approval.
SPAIN:
Spain’s health minister, Salvador Illa said that he expects to receive the country’s first coronavirus vaccine doses in January.
During a press conference on Tuesday, Illa presented the government’s plan that will prioritize the most vulnerable, which includes about 2.5 million people. Nursing home residents and staff will be first, followed by the disabled and general health workers, he said.
Spain’s strategy aims to vaccinate a significant part of the population within the first six months of 2021, with the plan expected to be completed in three stages.
The first one will begin in January until March with a limited number of doses available, followed by a second stage from March until June, when authorities expect to increase the number of vaccinations with a final stage starting in June, which is expected to cover a wider segment of the population.
The health minister also said he was confident his government’s plan will be able to provide vaccines for the whole country, noting that they had signed agreements that should allow for 140 million doses to
“According to the agreements we have signed, we estimate that Spain will receive 140 million doses to immunize approximately 80 million people, obviously this (number) is higher than our country’s population”
He said that the vaccine won’t be mandatory and will be available free of charge.
US:
The first Americans could receive a coronavirus vaccine by December 11, according to Dr. Moncef Slaoui, the head of the government’s effort to develop a vaccine against Covid-19.
On Friday, Pfizer submitted an application to the US Food and Drug Administration for emergency use authorization for their Covid-19 vaccine candidate, and an FDA vaccine advisory committee is slated to meet December 10.
Slaoui told CNN that means, if approved, the vaccine could be rolled out the next day.
“Our plan is to be able to ship vaccines to the immunization sites within 24 hours from the approval, so I expect maybe on day two after approval on the 11th or the 12th of December,” he said.
UK:
The UK’s Health Screechy Matt Hancock said in a statement on Monday that its national health service would be “ready to deliver” the Covid-19 vaccine following regulatory approval.
“The NHS has vast experience in delivering widespread vaccination programs and an enormous amount of work has taken place to ensure we have the logistical expertise, transport and workforce to roll out a vaccine according to clinical priority, at the speed at which it can be manufactured,” Hancock said.
Britain is expected to receive a total of 40 million doses of the Pfizer/BioNTech vaccine by the end of 2021 — which is “enough to vaccinate up to a third of the population, with the majority of doses anticipated in the first half of next year,” according to a statement from the Department of Health on Monday.
The Pfizer/BioNTech vaccine will only be authorized for supply by the Medicines and Healthcare products Regulatory Agency (MHRA) “if it meets strict standards of quality, safety, and effectiveness, and if they are satisfied the vaccine can be consistently manufactured,” it said.
You may also like
-
UK coronavirus variant has been reported in 86 countries, WHO says
-
NASA technology can help save whale sharks says Australian marine biologist and ECOCEAN founder, Brad Norman
-
California Twentynine Palms: Explosives are missing from the nation’s largest Marine Corps base and an investigation is underway
-
Trump unhappy with his impeachment attorney’s performance, sources say
-
Lunar New Year 2021: Ushering in the Year of the Ox

